Auteur: Munir Karjaghli, Kaouther Saihi
Date: 22.05.2023
In this article, we compare the various adaptive support ventilation modes available on the market.

Adaptive Support Ventilation (ASV)
Adaptive Support Ventilation® (ASV®) was the first commercially available adaptive ventilation mode to use an optimal targeting schema (
ASV is a closed‑loop ventilation mode that combines adaptive pressure‑controlled ventilation for passive patients, and adaptive pressure‑support ventilation for spontaneously breathing patients. This mode has the advantages of optimizing the patient's work and force of breathing, shortening the duration of mechanical ventilation supporting weaning and finally, reducing the workload of ICU staff while improving patient safety and comfort.
Principle of operation
The ASV algorithm was originally based on the lowest work of breathing according to Otis' equation (
To reduce the tidal volume (and consequently the driving pressure), the ASV algorithm was modified in 2017 to incorporate Mead's equation for the lowest force of breathing (


ASV 1.1
This modification resulted in the new version 1.1, which is the default selection on all Hamilton Medical ventilators. The ASV 1.1 algorithm selects the optimal frequency at which the breathing power and force are lowest and thus delivers ventilation at a lower mechanical power (MP) (
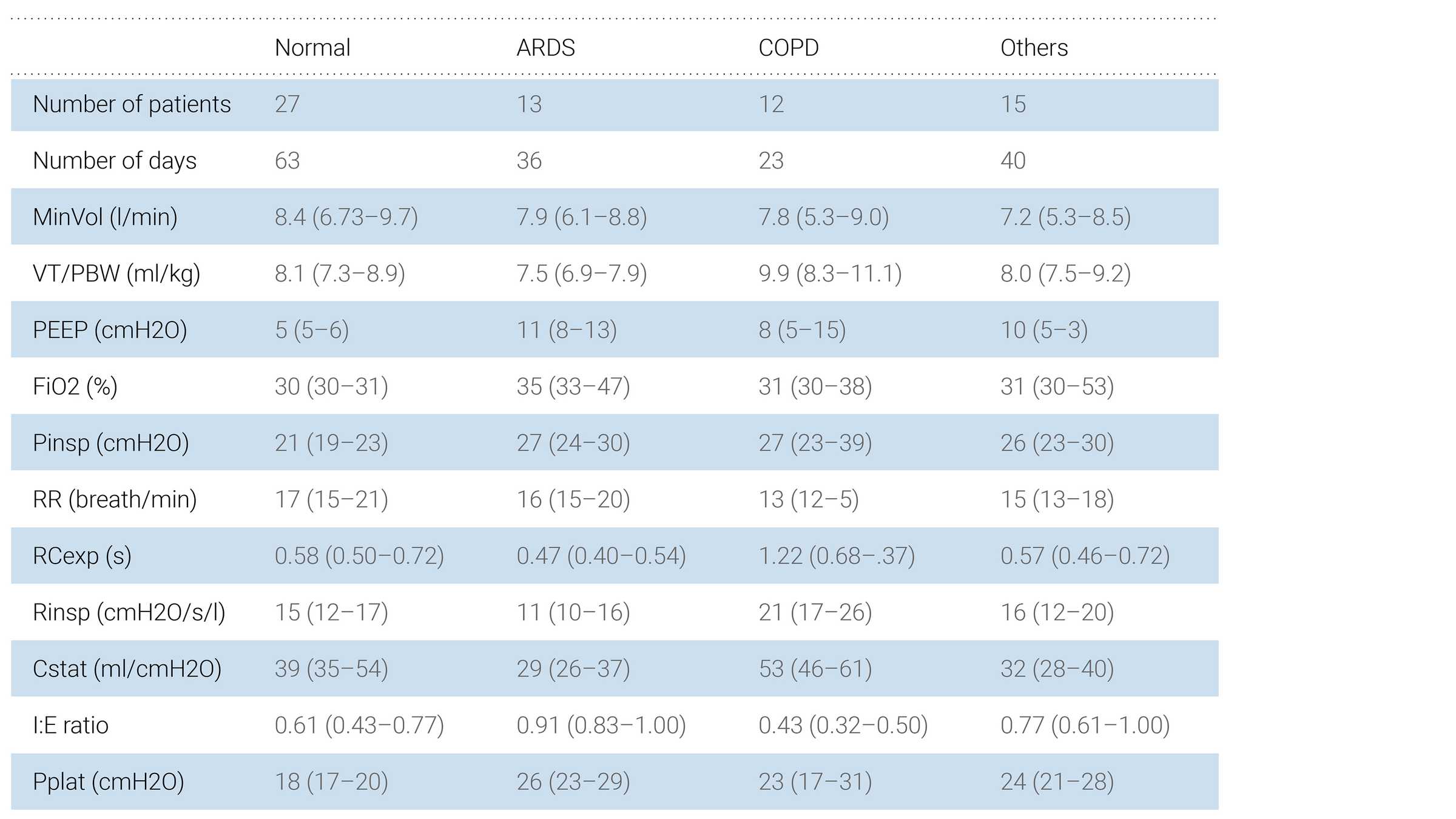
ASV 1.1 evaluates the patient's respiratory mechanics by measuring the expiratory time constant (RCexp) (
Recently, various other manufacturers have released adaptive ventilation modes similar to ASV 1.0, including “Adaptive Ventilation Mode” (IMT, Buchs, Switzerland), “Work of Breathing Optimized Ventilation” (Salvia Medical, Kronberg, Germany), and “Adaptive Minute Ventilation” (Mindray, Shenzhen, China). All these modes select a combination of RR and VT according to Otis’ equation and may therefore deliver a large VT, as observed in ASV by Dongelmans et al. (
AVM and AVM2 from IMT
The AVM algorithm was originally based on the lowest work of breathing according to Otis' equation as in the ASV mode. However, it was then modified in AVM2 to incorporate the concept of mean inspiratory power, whereby the optimal frequency is selected for the lowest inspiratory power. Inspiratory power is defined as the sum of the resistive and tidal power that is transmitted from the ventilator to the patient, assuming intrinsic PEEP is equal to zero.
AVM2 was announced in 2017 (
Comparison of targeting schemes
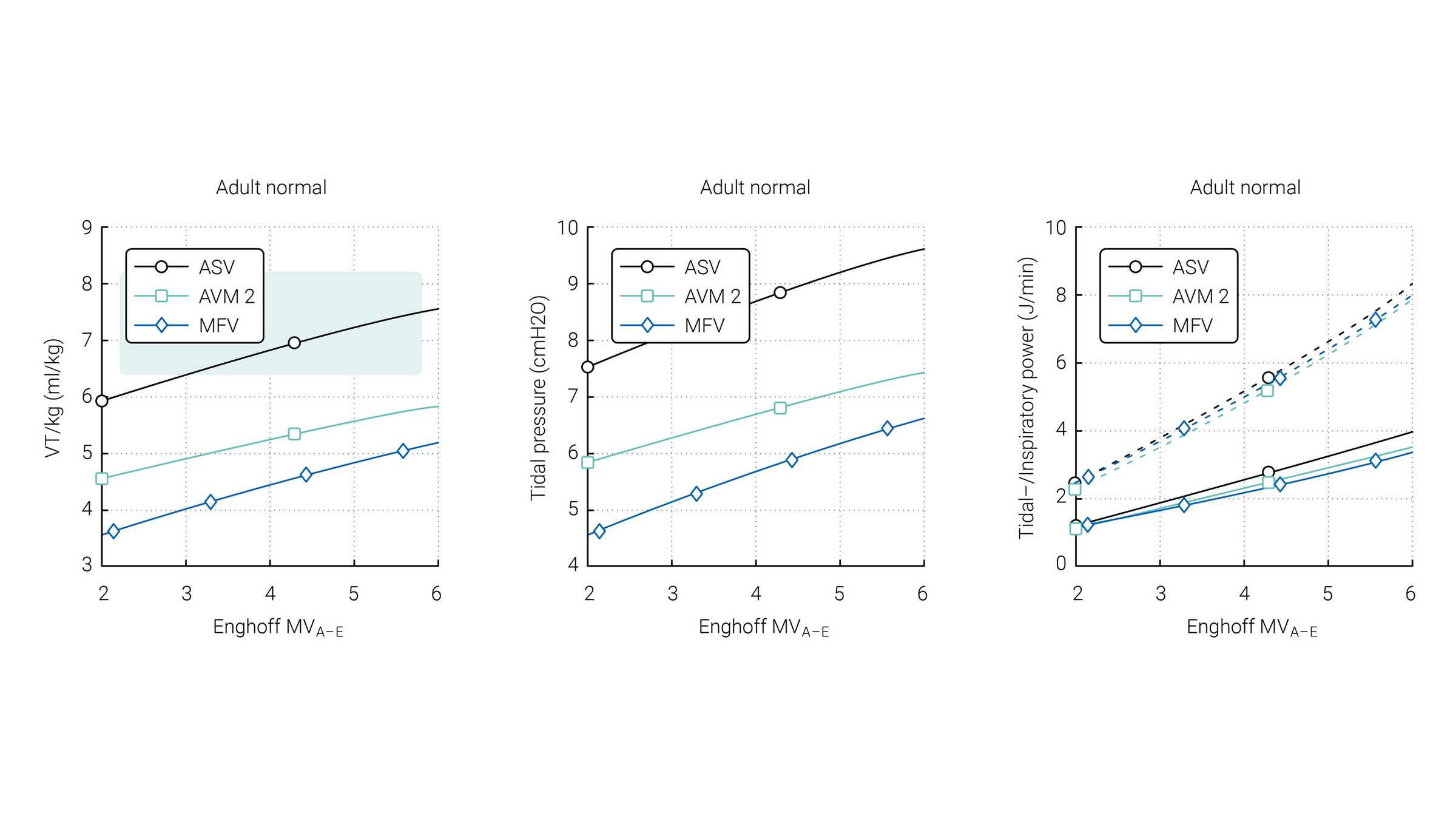
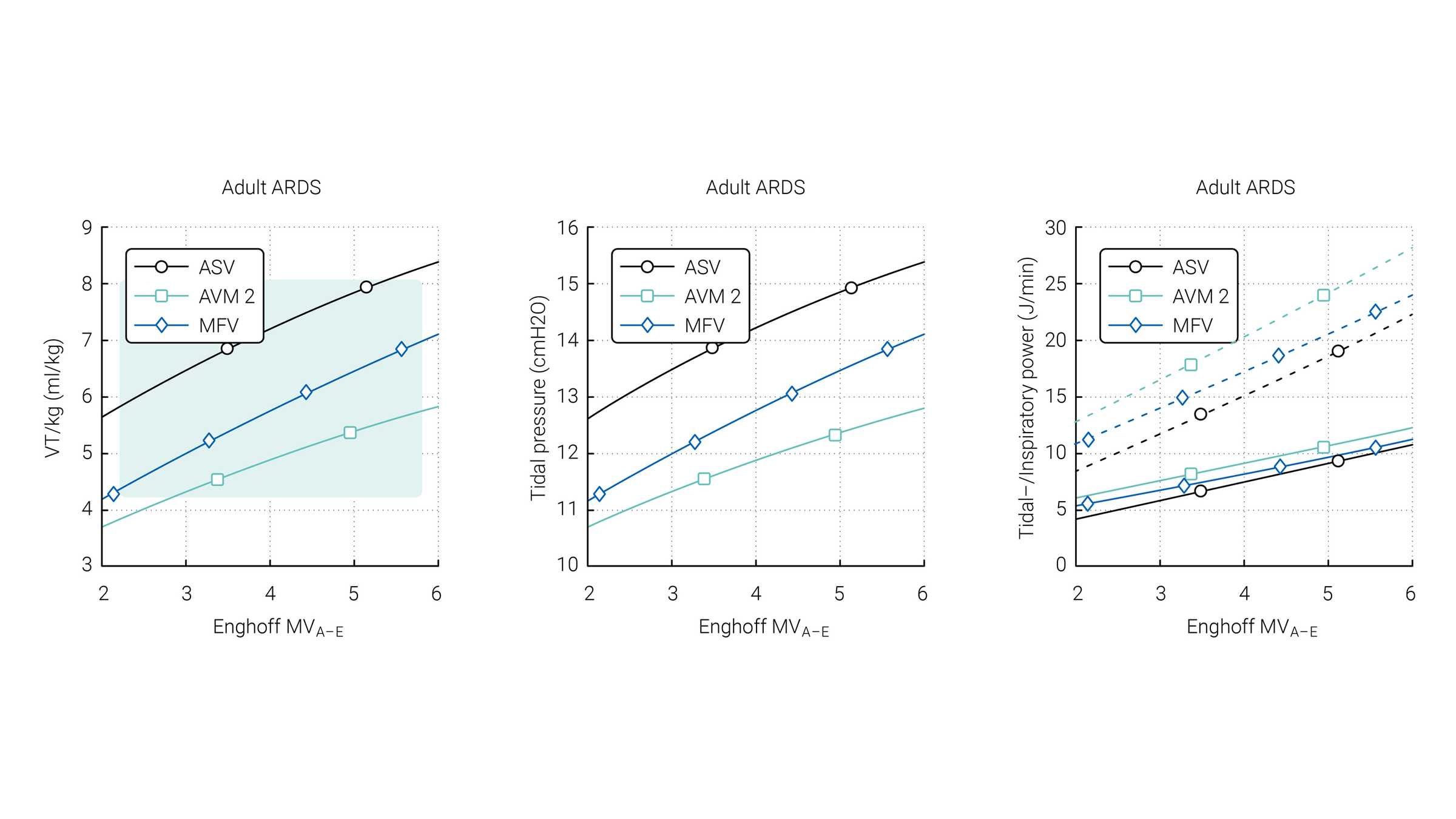
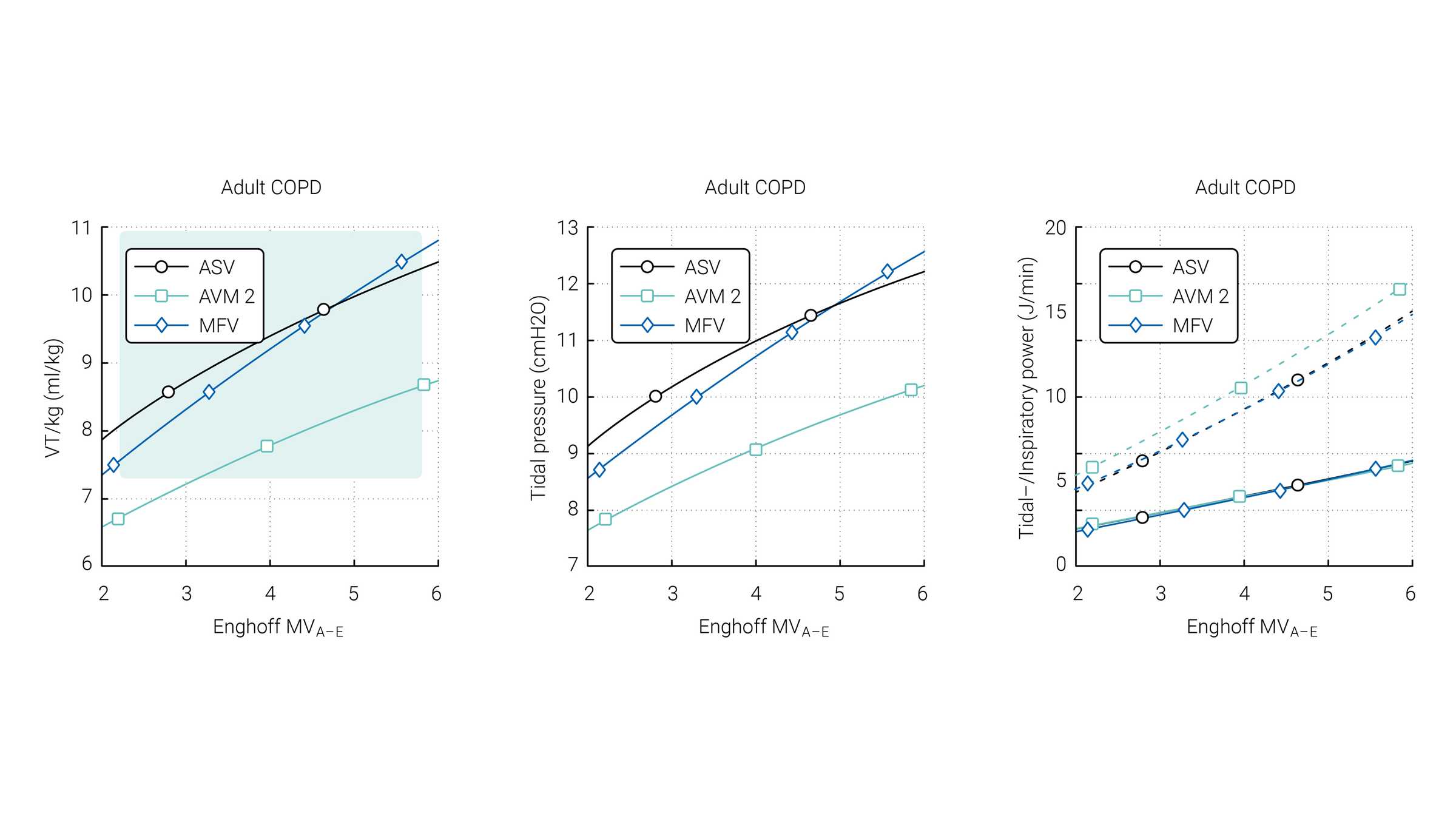
Van der Staay and Chatburn performed a comparison of three targeting schemes during selected simulation scenarios with ASV 1.0, AVM2, and MFV (
The green area highlights the range which is normally used for these patients according to Arnal et al. (



Evidence
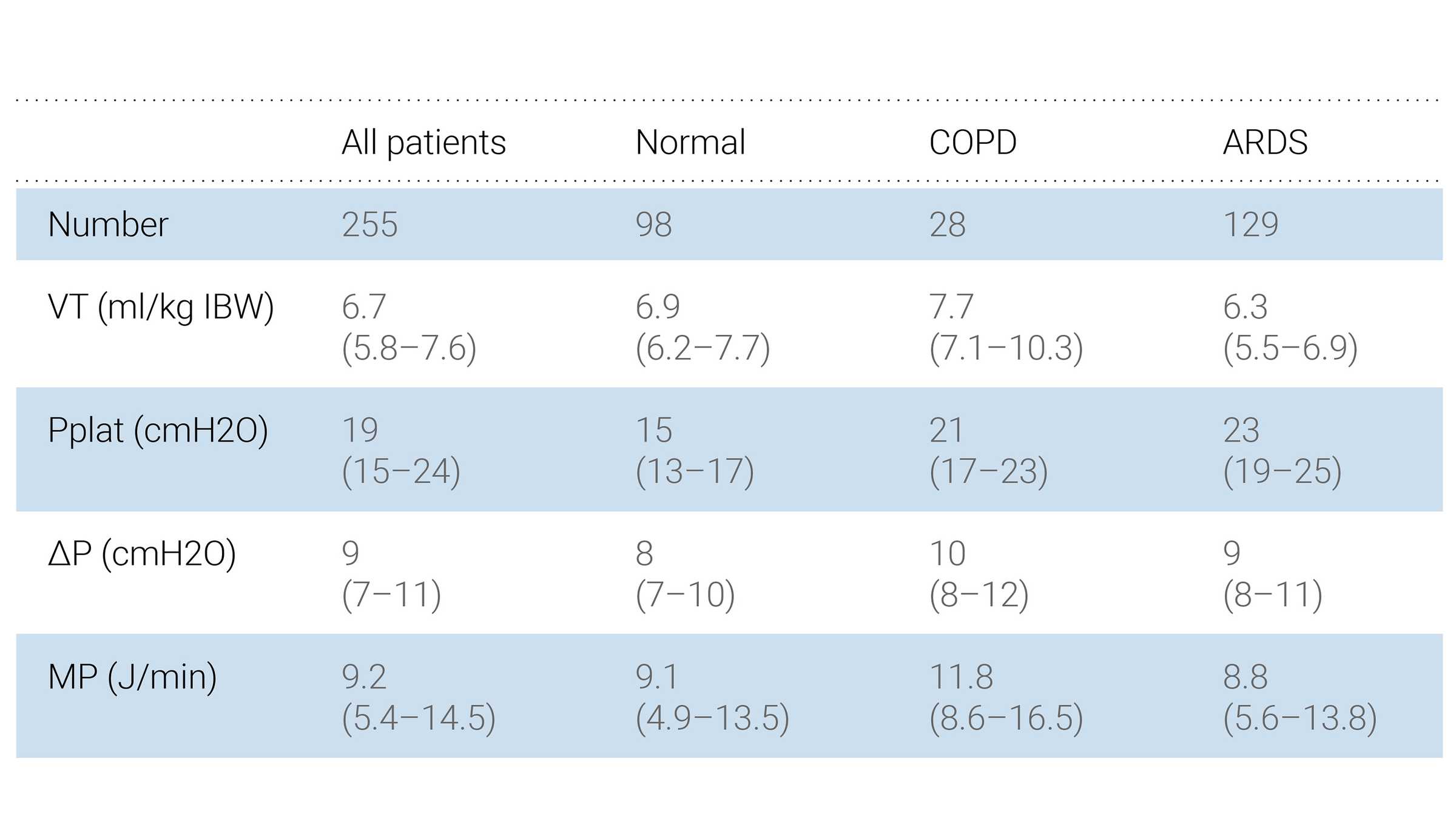
Several studies have been conducted to investigate the benefits of ASV 1.1 over conventional modes. These studies show that ASV 1.1 can select individualized VT‑RR combinations, and reduce the metabolic load and mechanical power delivered to the patient when compared to conventional ventilation modes (
Notes en bas de page
Références
- 1. Mireles‑Cabodevila E, Diaz‑Guzman E, Heresi GA, Chatburn RL. Alternative modes of mechanical ventilation: a review for the hospitalist [published correction appears in Cleve Clin J Med. 2009 Aug;76(8):445]. Cleve Clin J Med. 2009;76(7):417‑430. doi:10.3949/ccjm.76a.08043
- 2. OTIS AB, FENN WO, RAHN H. Mechanics of breathing in man. J Appl Physiol. 1950;2(11):592‑607. doi:10.1152/jappl.1950.2.11.592
- 3. Sulemanji D, Kacmarek R (2010) Adaptive support ventilation: an inappropriate mechanical ventilation strategy for acute respiratory distress syndrome? Anesthesiology 111(5):1295‑1296
- 4. Arnal JM, Garnero A, Novonti D, et al. Feasibility study on full closed‑loop control ventilation (IntelliVent‑ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study. Crit Care. 2013;17(5):R196. Published 2013 Sep 11. doi:10.1186/cc12890
- 5. Dongelmans DA, Paulus F, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Adaptive support ventilation may deliver unwanted respiratory rate‑tidal volume combinations in patients with acute lung injury ventilated according to an open lung concept. Anesthesiology. 2011;114(5):1138‑1143. doi:10.1097/ALN.0b013e31820d8676
- 6. MEAD J. The control of respiratory frequency. Ann N Y Acad Sci. 1963;109:724‑729. doi:10.1111/j.1749‑6632.1963.tb13500.x
- 7. Ceylan G, Topal S, Atakul G, et al. Randomized crossover trial to compare driving pressures in a closed‑loop and a conventional mechanical ventilation mode in pediatric patients. Pediatr Pulmonol. 2021;56(9):3035‑3043. doi:10.1002/ppul.25561
- 8. Wendel Garcia PD, Hofmaenner DA, Brugger SD, et al. Closed‑Loop Versus Conventional Mechanical Ventilation in COVID‑19 ARDS. J Intensive Care Med. 2021;36(10):1184‑1193. doi:10.1177/08850666211024139
- 9. Arnal JM, Saoli M, Garnero A. Airway and transpulmonary driving pressures and mechanical powers selected by INTELLiVENT‑ASV in passive, mechanically ventilated ICU patients. Heart Lung. 2020;49(4):427‑434. doi:10.1016/j.hrtlng.2019.11.001
- 10. Buiteman‑Kruizinga LA, Mkadmi HE, Schultz MJ, Tangkau PL, van der Heiden PLJ. Comparison of Mechanical Power During Adaptive Support Ventilation Versus Nonautomated Pressure‑Controlled Ventilation‑A Pilot Study. Crit Care Explor. 2021;3(2):e0335. Published 2021 Feb 15. doi:10.1097/CCE.0000000000000335
- 11. Brunner JX, Laubscher TP, Banner MJ, Iotti G, Braschi A. Simple method to measure total expiratory time constant based on the passive expiratory flow‑volume curve. Crit Care Med. 1995;23(6):1117‑1122. doi:10.1097/00003246‑199506000‑00019
- 12. van der Staay M, Remus C (2017) Adaptive ventilation mode 2
- 13. Becher T, Adelmeier A, Frerichs I, Weiler N, Schädler D. Adaptive mechanical ventilation with automated minimization of mechanical power‑a pilot randomized cross‑over study. Crit Care. 2019;23(1):338. Published 2019 Oct 30. doi:10.1186/s13054‑019‑2610‑7
- 14. van der Staay M, Chatburn RL. Advanced modes of mechanical ventilation and optimal targeting schemes. Intensive Care Med Exp. 2018;6(1):30. Published 2018 Aug 22. doi:10.1186/s40635‑018‑0195‑0
- 15. Arnal JM, Garnero A, Saoli M, Chatburn RL. Parameters for Simulation of Adult Subjects During Mechanical Ventilation. Respir Care. 2018;63(2):158‑168. doi:10.4187/respcare.05775
- 16. Chen YH, Hsiao HF, Hsu HW, Cho HY, Huang CC. Comparisons of Metabolic Load between Adaptive Support Ventilation and Pressure Support Ventilation in Mechanically Ventilated ICU Patients. Can Respir J. 2020;2020:2092879. Published 2020 Jan 28. doi:10.1155/2020/2092879