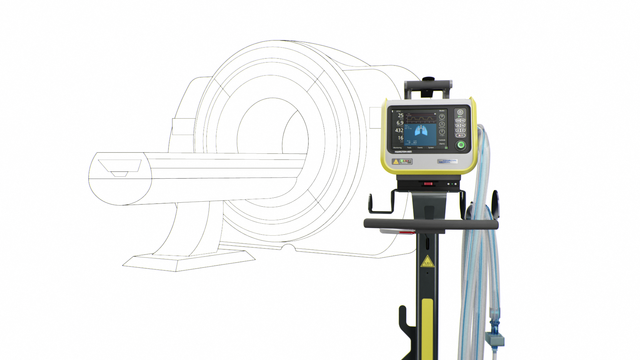
Our MRI specialist. For high‑end ventilation during MRI procedures

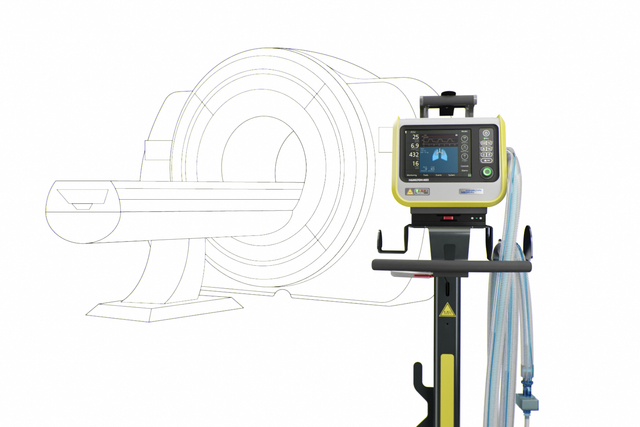
Our MRI specialist. For high‑end ventilation during MRI procedures

Walk with me! From the patient room to the MRI suite

Continuous ventilation therapy. Same mode and same settings as at the bedside

Always on the lookout. Your magnetic field navigator
The TeslaSpy helps you to keep the ventilator at a safe distance from the MRI scanner.

Two strong arms. For the extra‑long breathing circuits
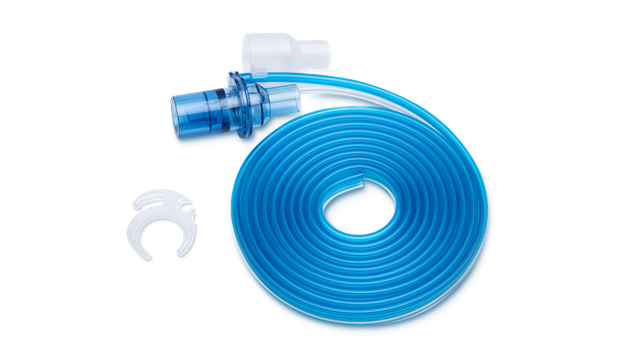
Choose between a range of breathing circuits in different lengths suitable for the MRI suite.

Stay! Locked in place automatically
The auto‑lock brakes on the trolley automatically lock in place, preventing the ventilator from accidentally rolling toward the MRI scanner.

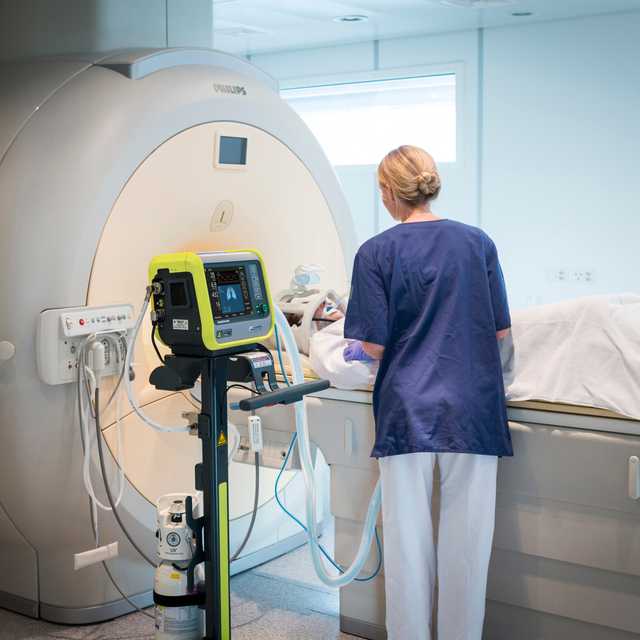
Come closer! MR‑Conditional up to 50 mT
The HAMILTON‑MR1 allows you to get close to the MRI scanner, because it is designed to withstand a static magnetic field of up to 50 mT. In comparison to ventilators with weaker shielding from the magnetic field, it gives you more freedom when positioning the device, a wider range of movement, and greater flexibility for your workflow and patient setup.
Closer proximity to the scanner also means you can use shorter breathing circuits. The advantage is their smaller compressible volume, which can contribute to better overall ventilation quality.

Click on, click off. For an easy transition
The quick‑release button on the transport kit allows you to detach the device from the trolley with just the push of a button. And it reattaches just as easily.
Want to see more?
Explore the 3D model
Discover the HAMILTON‑MR1 from every angle and click on the hotspots to learn more.
For quick details
- Standard
- Option
- Not available
| Patient groups | Adult/Ped, Neonatal |
|---|---|
| Dimensions (W x D x H) | 320 x 220 x 270 mm (ventilation unit) 630 x 630 x 1400 mm (incl. trolley) |
| Weight | 6.8 kg (15 lb) 21 kg (46.2 lb) with trolley |
| Monitor size and resolution | 8.4 in (214 mm) diagonal 640 x 480 pixels |
| Detachable monitor | |
| Battery operating time | 8 h with two batteries |
| Hot‑swappable battery | |
| Air supply | Integrated turbine |
| O2 connector | DISS (CGA 1240) or NIST |
| Connectivity | USB port |
| Loudness | 42 dB in normal operation |
| Volume controlled, flow controlled | |
|---|---|
| Volume targeted, adaptive pressure controlled | |
| Intelligent ventilation | ASV® |
| Noninvasive ventilation | |
| High flow | |
| Visualization of lung mechanics (Dynamic Lung) | |
|---|---|
| Visualization of the patient’s ventilator dependence | |
| Esophageal pressure measurement | |
| Capnography | |
| SpO2 monitoring |
| Recruitability assessment and lung recruitment (P/V Tool Pro) | |
|---|---|
| Patient‑ventilator synchronization (IntelliSync+) | |
| CPR ventilation | |
| Hamilton Connect Module |
| Remote connection to HAMILTON‑H900 humidifier | |
|---|---|
| Integrated IntelliCuff cuff pressure controller | |
| Integrated pneumatic nebulizer | |
| Integrated Aerogen nebulizer | |
| Compatibility with Sedaconda ACD‑S anesthetic delivery system |
For your patients
Intelligent ventilation solutions at a glance
ASV® ‑ Adaptive Support Ventilation®. For adaptation around the clock
The ventilation mode ASV continuously adjusts the respiratory rate, tidal volume, and inspiratory time breath by breath depending on the patient’s lung mechanics and effort ‑ 24 hours a day, from intubation to extubation.
Integrated nebulizer. For additional treatments
The integrated pneumatic nebulizer is fully synchronized with the timing of inspiration and expiration.
An integrated, synchronized Aerogen nebulizer is available as an option (
The delivery of a fine mist of drug aerosol particles helps you reverse bronchospasm, improve ventilation efficiency, and reduce hypercapnia (
CPR ventilation. For lifesavers
CPR ventilation adapts the ventilator settings during rescucitation. It supports the CPR workflow with quick access to preconfigurable settings, adequate alarm and trigger adjustment, and CPR‑timer display.
The main monitoring parameters and curves relevant to CPR ventilation are also displayed.
Vent Status panel. For those who are ready to wean
The Vent Status panel displays six parameters related to the patient’s ventilator dependence, including oxygenation, CO2 elimination, and patient activity.
A floating indicator moving up and down within each column shows the current value for a given parameter.
Dynamic Lung panel. For visual people
The Dynamic Lung panel shows you a graphic real‑time representation of the following important monitoring data:
- Compliance and resistance
- Patient triggering
- SpO2
- Pulse rate
High‑performance noninvasive ventilation. For mask-wearers
The noninvasive ventilation modes deliver pressure‑supported, flow‑cycled spontaneous breaths (NIV and NIV‑ST mode) and pressure‑controlled, time‑cycled mandatory breaths (NIV‑ST).
Compared to ventilators using compressed air, our turbine‑driven ventilators are capable of providing higher peak flow rates. This guarantees optimal performance even with large leaks.
Configurable loops and trends. For statisticians
The ventilator can display a dynamic loop based on a selected combination of monitored parameters. With the trend function, you can see trending information displayed for the monitoring parameters and time frame of your choice.
The device continually stores the monitored parameters in its memory, even when in Standby.
nCPAP modes. For the little ones
With the nCPAP mode, the patient is supported with a continuous positive airway pressure. In our flow‑controlled devices, the desired CPAP value is set via the respiratory gas flow. In order to compensate for any leakage that occurs, e.g. via the mouth or at the nose, the LeakAssist function can be activated. A predefined pressure can then be targeted with additional respiratory gas flow.
For you

Preassembled. And ready to use
Our preassembled breathing circuit sets include the essential consumables to operate the ventilator, conveniently packaged in one single bag.
All our essential consumables are specially developed for Hamilton Medical ventilators with guaranteed manufacturer quality.

Less knob‑turning. More adaptations to your patient
To manage ventilation you usually have to set multiple parameters, such as pressure, volume, inspiratory and expiratory triggers, cuff pressure, and more. And each time your patient's condition changes, you have to make one or even several readjustments.
To simplify this process and reduce the knob‑turning, we have created a range of solutions:
Adaptive Support Ventilation (ASV) is a ventilation mode that provides continuous adaptation of respiratory rate, tidal volume, and inspiratory time, depending on the patient’s lung mechanics and effort. ASV has been shown to shorten the duration of mechanical ventilation in various patient populations with fewer manual settings (
Conventional solutions for cuff pressure management require you to monitor and adjust cuff pressure by hand.
IntelliCuff secures your patient’s airway (

Help is near! On‑screen troubleshooting
Whenever there is a problem, the ventilator alerts you using the alarm lamp, sound, and message bar.
The on‑screen help offers you suggestions on how to resolve the alarm.

Farewell ventilator! Tools to implement your weaning protocols
We want our ventilator to leave your patient’s side as quickly as possible. That is why we provide you with tools to help you implement your weaning protocol.
These include visual aids and ventilation modes designed to encourage spontaneous breathing.
Get the hang of it! Learning paths and educational content
Our online Academy offers easy‑to‑follow learning paths to familiarize you with Hamilton Medical products and technologies as quickly as possible.
For the future

Constant evolution. Expanding your ventilator’s capabilities
We are constantly working on further evolving our products. New features are added and existing features improved to ensure you always have access to the latest ventilation technology over your ventilator’s lifetime.


Know one, know them all. A universal user interface
Whether it is in the ICU, in the MRI suite, or during transport, the user interface of all Hamilton Medical ventilators works in the same way.
Our Ventilation Cockpit integrates complex data into intuitive visualizations.
For the complete solution
Fully integrated accessories
We develop our accessories for the highest possible patient safety and ease of use in mind. Whenever possible, we integrate them with our ventilators to simplify operation of the complete ventilator system.
Our consumables

Talk to our experts. Let's discuss your needs
Our team of Ventilation Geeks is happy to assist you in choosing the perfect ventilator for your clinical care setting and help you meet your therapy goals. Get a personalized price quote or schedule a callback for more information.
For more information
HAMILTON‑MR1 brochure Software version 3.0.x USA Version
HAMILTON‑MR1 Technical specification for SW version 3.0.x USA Version
The new HAMILTON‑C1/T1/MR1 SW3.0.x brochure USA Version
Transport and storage solutions for HAMILTON‑C1/T1/MR1
References
- 1. Kirakli C, Naz I, Ediboglu O, Tatar D, Budak A, Tellioglu E. A randomized controlled trial comparing the ventilation duration between adaptive support ventilation and pressure assist/control ventilation in medical patients in the ICU. Chest. 2015;147(6):1503‑1509. doi:10.1378/chest.14‑2599
- 2. Tam MK, Wong WT, Gomersall CD, et al. A randomized controlled trial of 2 protocols for weaning cardiac surgical patients receiving adaptive support ventilation. J Crit Care. 2016;33:163‑168. doi:10.1016/j.jcrc.2016.01.018
- 3. Zhu F, Gomersall CD, Ng SK, Underwood MJ, Lee A. A randomized controlled trial of adaptive support ventilation mode to wean patients after fast‑track cardiac valvular surgery. Anesthesiology. 2015;122(4):832‑840. doi:10.1097/ALN.0000000000000589
- 4. Chenelle CT, Oto J, Sulemanji D, Fisher DF, Kacmarek RM. Evaluation of an automated endotracheal tube cuff controller during simulated mechanical ventilation. Respir Care. 2015;60(2):183‑190. doi:10.4187/respcare.03387
- 100. Dhand R. New frontiers in aerosol delivery during mechanical ventilation. Respir Care. 2004;49(6):666‑677.
- 101. Waldrep JC, Dhand R. Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Curr Drug Deliv. 2008;5(2):114‑119. doi:10.2174/156720108783954815
Footnotes
- a. Not available in all markets
- b. Only available for HAMILTON‑C6/G5/S1