Author: Jean‑Michel Arnal, Senior Intensivist, Hopital Sainte Musse, Toulon, France
Date of first publication: 04.12.2023
A recent publication documents results from the largest international study so far to investigate the management and outcomes of weaning in ICU patients.

Takeaway messages
- Despite the impact of weaning on patient outcomes and cost, the weaning process is still poorly understood.
- The WEAN SAFE study is the largest international study to investigate management practices and outcomes in a real‑world population and included 5,869 ICU patients from 50 countries.
- The study confirmed that both the patient’s physiological characteristics and weaning practices contribute to weaning failure.
- Deep sedation at the time of the separation attempt and delays between meeting the weaning eligibility criteria and the first separation attempt were both independently associated with weaning failure.
What is behind the WEAN SAFE study?
Weaning from mechanical ventilation refers to the process of liberating the patient from the ventilatory support and artificial airway. Weaning represents a significant amount of the total invasive ventilation duration in ICU patients, and is associated with both short‑ and long‑term outcomes (
Patient characteristics and weaning eligibility criteria
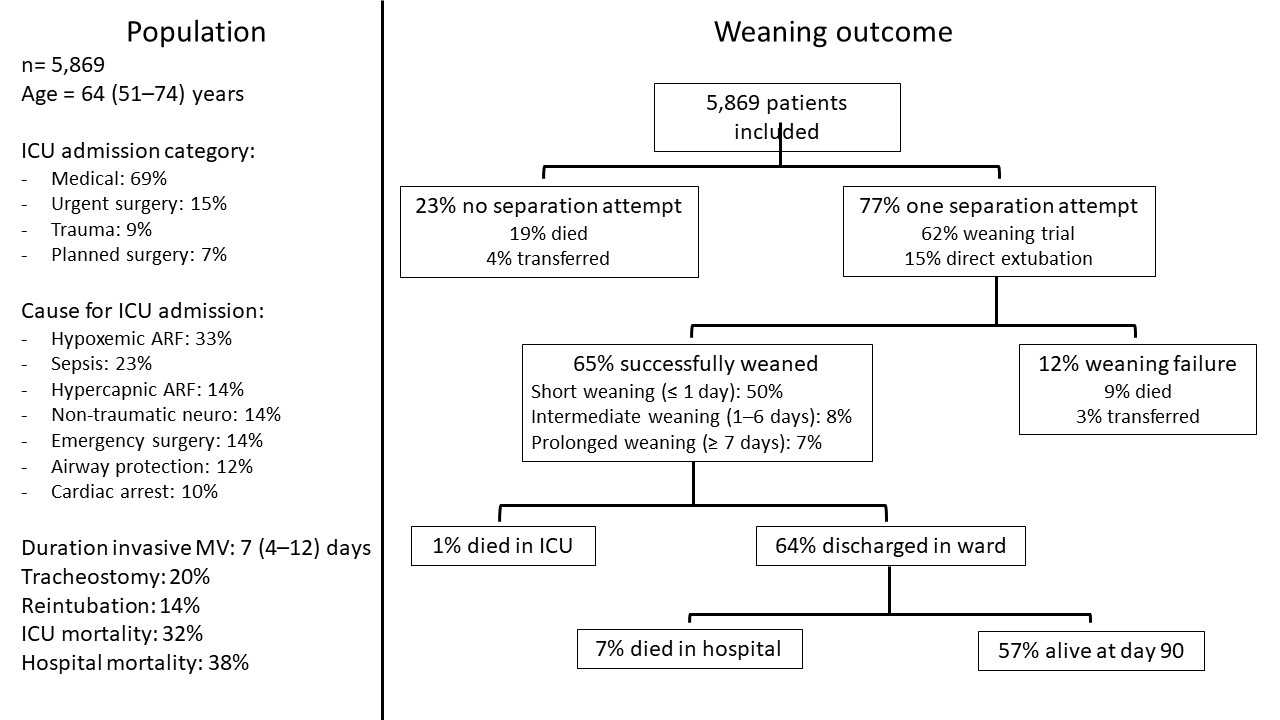
All patients over 16 years of age and mechanically ventilated in the ICU were screened, and were included if they were still receiving invasive mechanical ventilation two calendar days after intubation. The weaning eligibility criteria were FiO2 below 50%, PEEP below 10 cmH2O, no or low doses of vasopressors, and no paralyzing agent. The level of consciousness was not taken into account for assessing weaning eligibility, as it is a criterion for extubation. The start of weaning was defined as the first weaning trial, whatever the method used, or the first extubation without a weaning trial. Weaning success was defined as extubation or disconnection from the tracheostomy tube without death or reintubation within the following seven days. Patient’s characteristics and weaning outcomes are presented in Figure 1.

Time until first attempt and weaning duration
More than 90% of the included patients presented signs of spontaneous activity and met the weaning eligibility criteria at a median of three days following tracheal intubation. The median time from meeting the weaning eligibility criteria and the first attempt at separation was 1 day, but in 22% of patients this delay was prolonged to more than 5 days. Of the patients who were weaned successfully, the weaning process was short in 77% (≤ 1 day), of intermediate duration in 12% (2‑6 days), and prolonged in 11% (≥ 7 days).
The demographic factors associated with a delay of more than 1 day between meeting the eligibility criteria and the first separation attempt included frailty, admission for trauma, and a non‑traumatic neurological event. Critical illness severity as measured by the SOFA score was also associated with a prolonged delay. The continuous use of neuromuscular blockades and use of moderate or deep sedation on the first day of fulfilling the weaning criteria were also associated with a delayed separation attempt.
Factors associated with failure
Factors associated with weaning failure were older age, frailty, immunocompromised status, severity of critical illness, cardiac arrest, non‑traumatic neurological event, pre‑existing limitation of care, and severe respiratory dysfunction. The presence of deep sedation at the time of the separation attempt, and a delay between meeting the eligibility criteria and the first separation attempt were independently associated with weaning failure. A sensitivity analysis was performed including the number of beds in the ICU and the same results were found.
What have we learned?
Weaning failure and delayed weaning are a burden for the patient, family, and healthcare system. The WEAN SAFE study confirms that both the patient’s physiological characteristics and weaning practices contribute to weaning failure. One major driver behind delays to the first separation attempt that eventually increase weaning failure is the sedation management at the time of meeting the weaning eligibility criteria. Every effort to prevent moderate and deep sedation at the time of assessing the weaning eligibility may have a positive impact, shortening the invasive mechanical ventilation duration and increasing the weaning success rate. Another lesson from the WEAN SAFE study is that a delay in performing the first weaning trial after patients have met the eligibility criteria is associated with weaning failure. Sometimes, this delay is due to the patient’s status and there is a good reason for not performing the weaning trial. On other occasions, however, the delay is due to under‑recognition of the patient’s readiness‑to‑wean or that caregivers are not available to perform the weaning trial in a timely manner. The recent shortages of ICU nurses, respiratory therapists, and physicians are impacting on the organization in the ICU and may affect the weaning process.
One point of discussion is whether we should wait for the patient to be awake and fully responsive to perform the first weaning trial. The authors support the approach that the first weaning trial should be performed even if the extubation criteria (consciousness, cough, etc.) are not fulfilled. By doing this, the clinician can recognize if the patient would be ready to wean and stop sedation accordingly (
How can ventilators support the process?
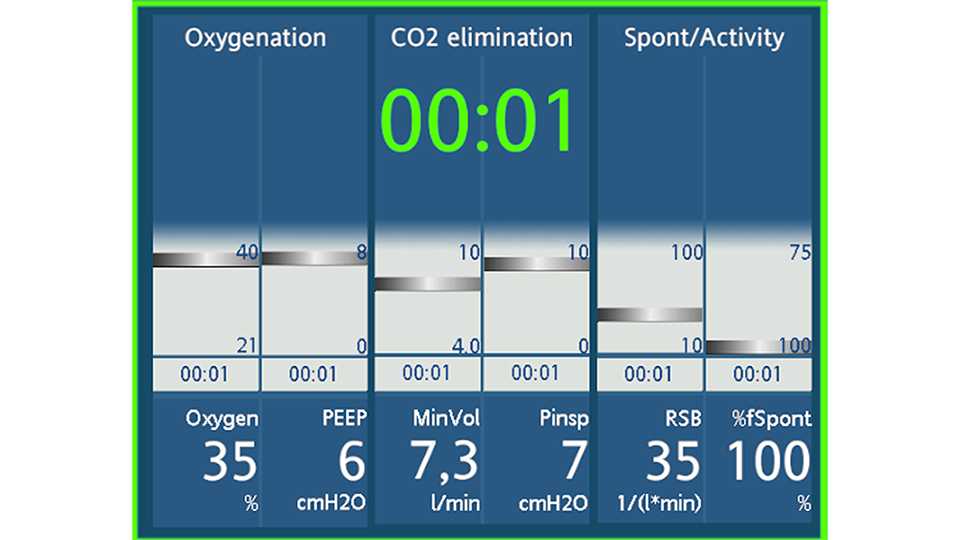
Hamilton Medical ventilators provide some tools to support clinicians in the weaning process and, in particular, to prevent delays in recognizing the readiness‑to‑wean criteria and performing a weaning trial. The Vent Status panel is a simple display of the weaning eligibility criteria (see Figure 2). Even without a lot of cognitive involvement or deep knowledge, one look at the screen can tell the clinician whether that patient is ready for a weaning trial. If vasopressor doses are low, the clinician is encouraged to stop sedation and perform a weaning trial.
For the patients ventilated with INTELLiVENT‑ASV, the Quick Wean function is an automatic weaning protocol to support the caregivers in the weaning process. Quick Wean can be activated as soon as the patient recovers spontaneous breathing, whatever the level of oxygen, pressure support, or sedation. The ventilator decreases oxygen, PEEP, and pressure support within the safety rules of the mode, screens the weaning eligibility criteria, and performs a weaning trial. The weaning trial aborts automatically if the physiological variables are outside of target ranges. The clinician has to manage the sedation and decide when it is time to extubate.

Conclusion
In conclusion, the WEAN SAFE study is a large, international, multicenter observational study that showed important variations in weaning practices and identified some modifiable factors to decrease weaning duration and increase weaning success.
Footnotes
References
- 1. Herridge MS, Azoulay É. Outcomes after Critical Illness. N Engl J Med. 2023;388(10):913‑924. doi:10.1056/NEJMra2104669
- 2. Pham T, Heunks L, Bellani G, et al. Weaning from mechanical ventilation in intensive care units across 50 countries (WEAN SAFE): a multicentre, prospective, observational cohort study [published correction appears in Lancet Respir Med. 2023 Mar;11(3):e25]. Lancet Respir Med. 2023;11(5):465‑476. doi:10.1016/S2213‑2600(22)00449‑0
- 3. Pham T, Heunks L, Bellani G, Brochard L, Laffey J; WEAN SAFE Investigators. WEAN SAFE and the definition of the first separation attempt ‑ Authors' reply. Lancet Respir Med. 2023;11(5):e44. doi:10.1016/S2213‑2600(23)00089‑9